Taking FIRDAPSE
How FIRDAPSE is supplied
FIRDAPSE is an oral medication that comes in 10-mg scored tablets. These tablets are supplied in bottles as well as blister packs, so you and your doctor can choose the right option for you.

How to take FIRDAPSE
FIRDAPSE is typically taken 3 to 4 times per day and can be taken with or without food. It’s important to take FIRDAPSE exactly as directed by your doctor.
*10-mg tablets represents adult dosing; please see full Prescribing Information for pediatric dosing information. Each patient’s dose is based on individual needs. Your doctor will work with you to find the right dose for you. Use a pill cutter when necessary to take the prescribed dose. Dosage should not exceed a maximum daily dose of 80 mg.
Sticking to the schedule
It is important to follow your prescription carefully. Take your FIRDAPSE dose on time and according to schedule.
If you miss a dose, skip it altogether and take your next dose at the scheduled time.
- Do not take 2 doses to make up for a missed dose!
- Take FIRDAPSE exactly as your doctor tells you to take it.
- If your dose is less than 5 mg, you have trouble swallowing tablets, or a feeding tube is needed, see the detailed Instructions for Use on how to take and prepare a suspension of FIRDAPSE.
What to expect
Some people may experience a rapid improvement in their symptoms when treated with FIRDAPSE. For others, it may take longer to reach the optimal therapeutic dosethe dose that provides the most symptom relief with the fewest side effects .
Your dose of FIRDAPSE will be customized by your doctor based on how well your treatment manages your symptoms and how well you tolerate the treatment. Once you start FIRDAPSE, your doctor may steadily increase your dose according to an established schedule. This process is called titration.
Pregnant and taking FIRDAPSE?
In conjunction with the FDA, Catalyst Pharmaceuticals has created a registry to collect information about the safety of FIRDAPSE use in the event of pregnancy. Patients with LEMS—even those not taking FIRDAPSE—may enroll. Contact the registry as soon as you learn that you are pregnant by calling 1-855-212-5856 or visiting www.firdapsepregnancystudy.com.
Help us track the safety of FIRDAPSE use during pregnancy. Contact the FIRDAPSE Pregnancy Registry by calling (855) 212-5856 or visiting
www.firdapsepregnancystudy.com.
The dose titration process for patients ≥ 6 years of age and weighing ≥ 45 kg*
It is important to provide regular feedback to your physician during this process so he or she can identify your optimal therapeutic dose.
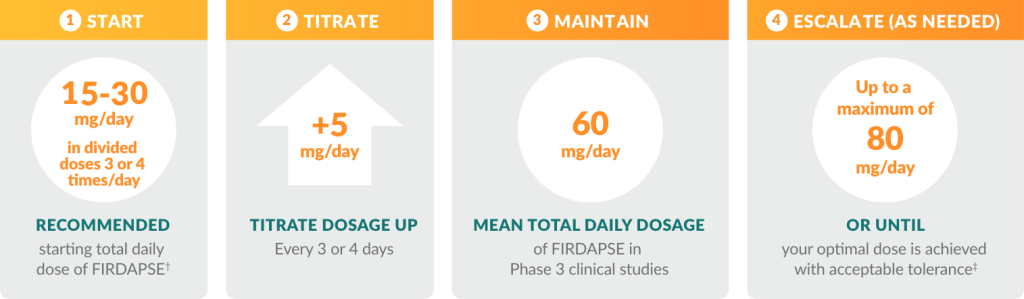
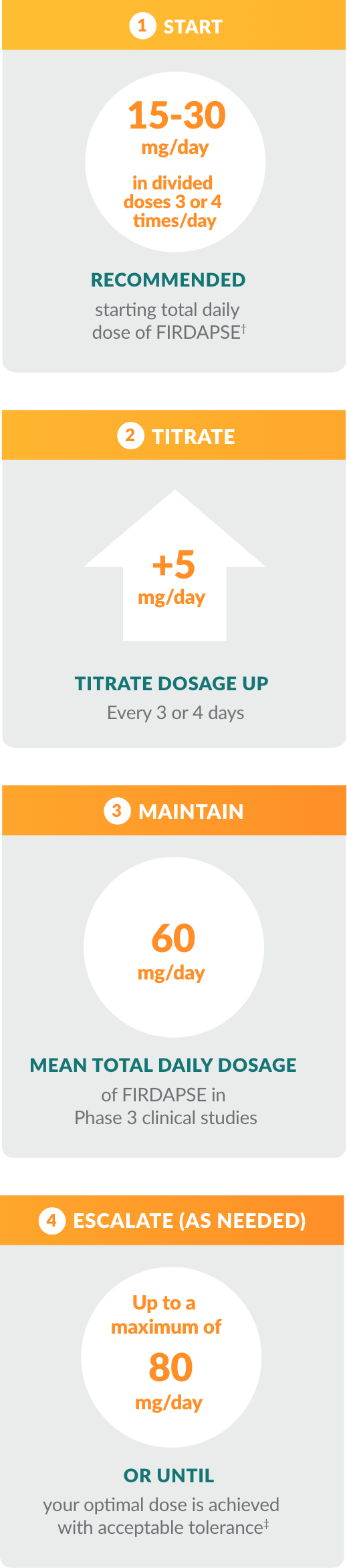
† The lowest starting initial daily dosage is recommended for adult and pediatric patients with kidney or liver impairment, or patients who slowly metabolize FIRDAPSE.
‡ For patients age 6 years and older weighing 45 kg or more, the maximum single dose for FIRDAPSE is 20 mg.



