What is LEMS?
Important facts about Lambert-Eaton myasthenic syndrome (LEMS)
LEMS IS a neuromuscular disorder (a condition that affects the nerves and muscles) that typically causes severe, debilitating, and progressive muscle weakness and fatigue.
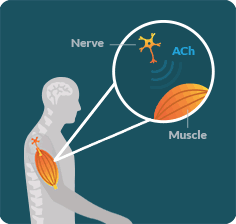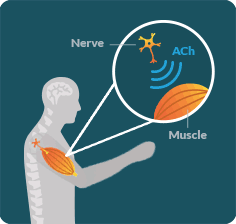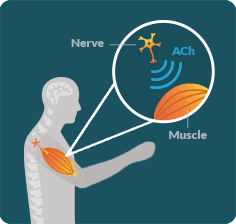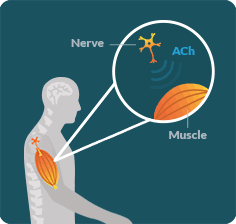
LEMS OCCURS WHEN the immune system disrupts communication between the nerves and muscles in an area known as the neuromuscular junction (NMJ).
LEMS DISRUPTS the ability of nerves to release an important chemical called acetylcholine (ACh). When ACh is not released properly, muscles lose the signal necessary for their full function. As a result, people with LEMS may struggle to walk or do everyday activities.
WHO IS AFFECTED BY LEMS?
SYMPTOMS OF LEMS include muscle weakness, especially in the legs and hips. This weakness may fluctuate from day to day. LEMS can make it very difficult to do everyday activities, such as walking, talking, or lifting objects. Some patients may have to use assistive equipment, such as a wheelchair or walker, to get around. If you suspect that you may have LEMS, talk to your doctor about the symptoms you’re experiencing and your possible treatment options.
SYMPTOM SELF-ASSESSMENT
Could your symptoms be a sign of LEMS?
TAKE A SHORT SELF-ASSESSMENT AND TALK TO YOUR DOCTOR TO SEE WHAT YOUR SYMPTOMS MAY BE TRYING TO TELL YOU
We’ll send you a summary of your responses, along with other helpful information that you can use when talking with your physician.
LEMS IS OFTEN FIRST DIAGNOSED AS ANOTHER CONDITION because its symptoms resemble other common diseases, including:
- Myasthenia gravis (MG)
- Multiple sclerosis (MS)
- Parkinson’s disease
- Amyotrophic lateral sclerosis (ALS)
- Guillain-Barré syndrome
Many people with LEMS struggle with symptoms for years before finally getting diagnosed. For that reason, it’s important to see a physician who has experience with diagnosing neuromuscular diseases.
Who treats LEMS?
If you think that you may be experiencing symptoms of LEMS, talk to your physician. If your physician suspects that you may have LEMS, he or she may refer you to a neurologist or neuromuscular specialist. These types of doctors specialize in neuromuscular diseases like LEMS. Find a LEMS physician nearest to you now.
Diagnostic tests your doctor may use
LEMS is typically diagnosed using one or more of the following ways:
Benefits of earlier diagnosis
An early, accurate diagnosis may help you in several ways:
MORE ABOUT LEMS AND CANCER
For some patients, their LEMS symptoms are an important early warning sign that may help save their lives. The timing of LEMS is variable: LEMS symptoms, including muscle weakness, can appear before a cancer diagnosis or even 5 years or more after cancer is discovered-and those symptoms can worsen at any time.
About 50% of people with LEMS have an underlying cancer—often small cell lung cancer
The majority of these cases occur in patients with a history of smoking
Your treating physician may recommend cancer screenings when you are diagnosed with LEMS and periodically thereafter.