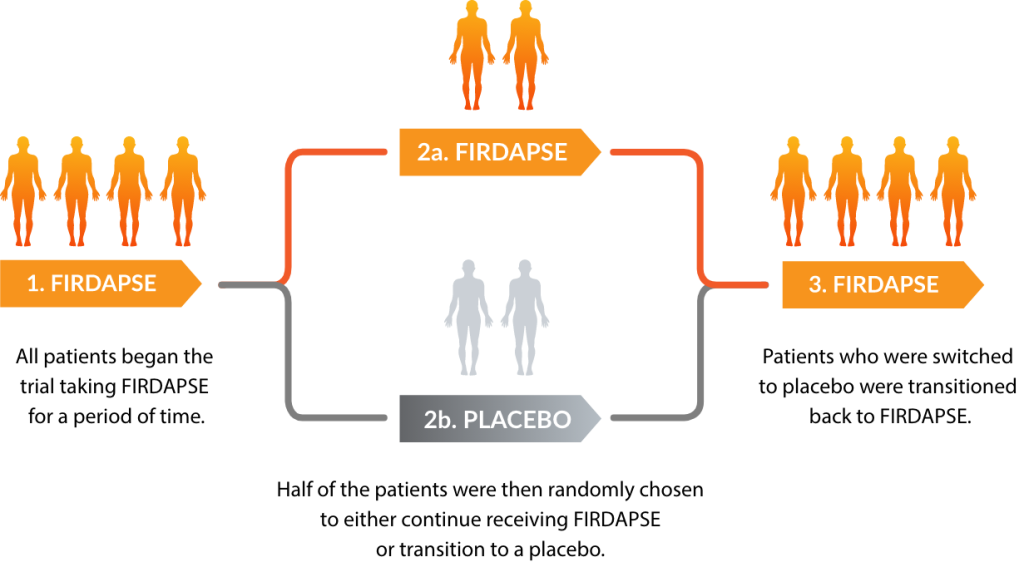
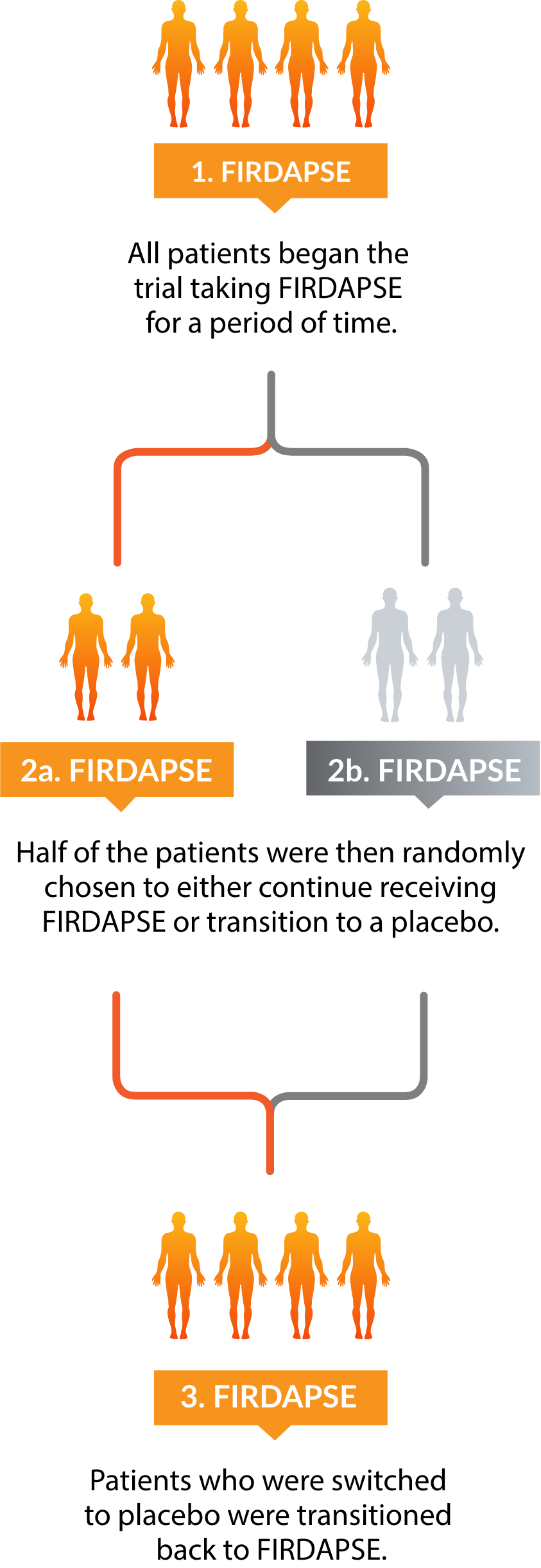
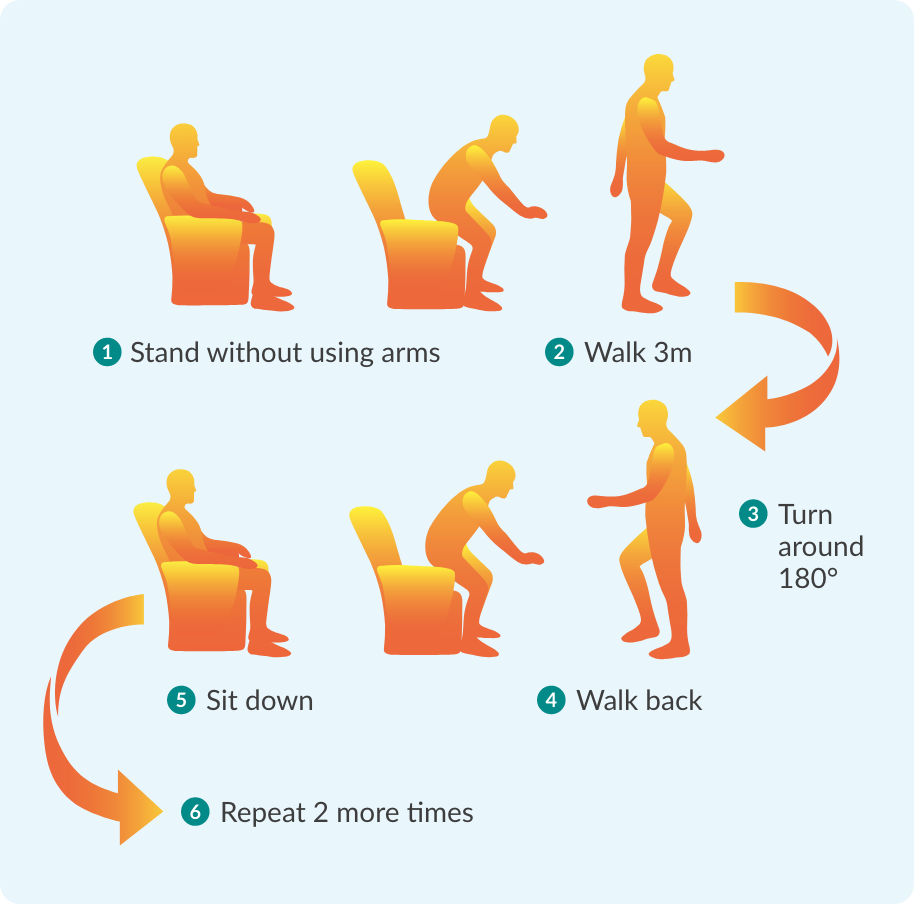
What is FIRDAPSE®?
FIRDAPSE is a prescription medicine used to treat Lambert-Eaton myasthenic syndrome (LEMS) in people 6 years of age or older. It is not known if FIRDAPSE is safe or effective in children less than 6 years of age.
IMPORTANT SAFETY INFORMATION
What is the most important information I should know about FIRDAPSE?
- FIRDAPSE can cause seizures.
- You could have a seizure even if you never had a seizure before.
- Do not take FIRDAPSE if you have ever had a seizure.
Stop taking FIRDAPSE and call your doctor right away if you have a seizure while taking FIRDAPSE.
Do not take FIRDAPSE if you:
- have ever had a seizure.
- are allergic to amifampridine phosphate, or another aminopyridine.
Before you take FIRDAPSE, tell your doctor about all of your medical conditions, including if you:
- are taking another aminopyridine, such as compounded 3,4-diaminopyridine (3,4-DAP)
- have had a seizure
- have kidney problems
- have liver problems
- are pregnant or plan to become pregnant. It is not known if FIRDAPSE will harm your unborn baby. You and your doctor will decide if you should take FIRDAPSE while you are pregnant.
- There is a registry for women who become pregnant during treatment with FIRDAPSE. The purpose of this registry is to collect information about your health and your baby’s health. Contact the registry as soon as you learn that you are pregnant, or ask your healthcare provider to contact for you by calling 855-212-5856 (toll free), contacting the Fax number 877-867-1874 (toll free), emailing the Pregnancy Coordinating Center at firdapsepregnancyregistry@ubc.com, or visiting the study website www.firdapsepregnancystudy.com.
- are breastfeeding or plan to breastfeed. It is not known if FIRDAPSE passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking FIRDAPSE.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take FIRDAPSE?
- If your dose is less than 5 mg, you have trouble swallowing tablets, or a feeding tube is needed, see the detailed Instructions for Use on how to take and prepare a suspension of FIRDAPSE.
- Take FIRDAPSE exactly as your doctor tells you to take it.
- Do not change your dose of FIRDAPSE.
- Do not stop taking FIRDAPSE without first talking to your doctor.
- FIRDAPSE tablets are scored and can be split if less than a full tablet is needed for you to get the right dose.
- FIRDAPSE can be taken with or without food.
- If you miss a dose of FIRDAPSE, skip that dose and take your next dose at your next scheduled dose time. Do not double your dose to make up the missed dose.
- Do not take FIRDAPSE together with other medicines known to increase the risk of seizures.
- If you take too much FIRDAPSE, call your doctor or go to the nearest hospital emergency room right away.
What are the possible side effects of FIRDAPSE?
FIRDAPSE may cause serious side effects, including:
- Seizures. See “What is the most important information I should know about FIRDAPSE?”
- Serious allergic reactions, such as anaphylaxis. FIRDAPSE can cause serious allergic reactions. Stop taking FIRDAPSE and call your doctor right away or get emergency medical help if you have:
- shortness of breath or trouble breathing
- swelling of your throat or tongue
- hives
- The most common side effects of FIRDAPSE include:
- tingling around the mouth, tongue, face, fingers, toes, and other body parts
- upper respiratory infection
- stomach pain
- nausea
- diarrhea
- headache
- increased liver enzymes
- back pain
- high blood pressure
- muscle spasms
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of FIRDAPSE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store FIRDAPSE?
- Store FIRDAPSE tablets at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Safely throw away FIRDAPSE tablets that are out of date or no longer needed.
- Store FIRDAPSE prepared oral suspension in the refrigerator between 36°F to 46°F (2°C to 8°C) between doses for up to 24 hours.
- Safely throw away unused FIRDAPSE oral suspension after 24 hours.
Keep FIRDAPSE and all medicines out of the reach of children.
General Information about the safe and effective use of FIRDAPSE
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use FIRDAPSE for a condition for which it was not prescribed. Do not give FIRDAPSE to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information, talk to your doctor or pharmacist. You can ask your pharmacist or doctor for information about FIRDAPSE that is written for health professionals.
What are the ingredients in FIRDAPSE?
Active ingredient: amifampridine
Inactive ingredients: calcium stearate, colloidal silicon dioxide, and microcrystalline cellulose.
Please see full Prescribing Information for additional Important Safety Information.